
* Visit
inorganicventures.com/tech/icp-operations/for additional information from this link
ulti-Element Standard Blends
Elemental and Matrix Compatibility
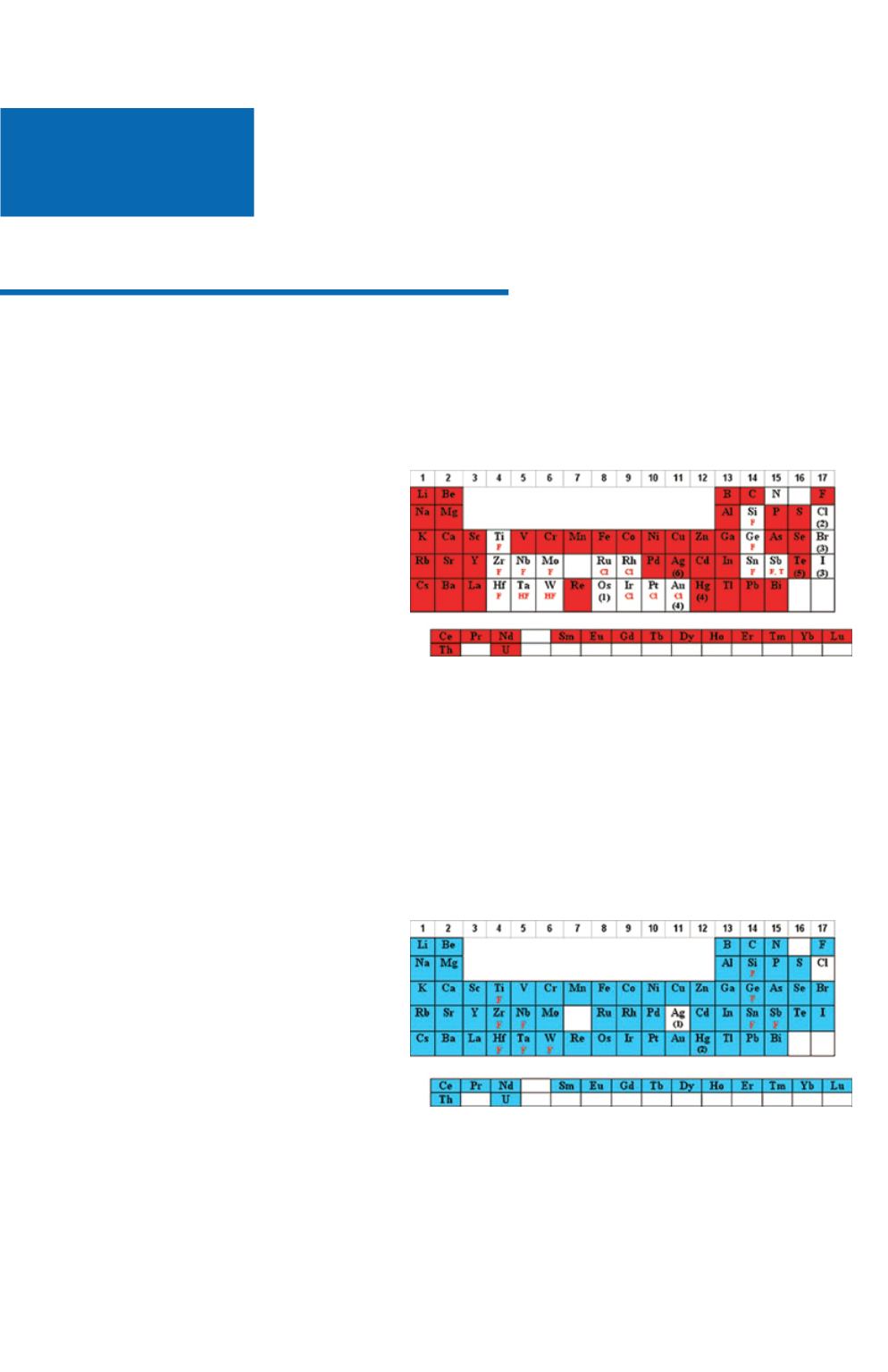
M
1
Nitric Acid Matrices
Most analysts prefer nitric acid (HNO
3
) matrices due to the solubility of the nitrates as well as its oxidizing ability and
the relative freedom from chemical and spectral interferences as compared to acids containing Cl, S, F, or P. In addition,
HNO
3
is very popular in acid digestion sample preparations.
The elements that are stable/soluble and commonly diluted in aqueous/HNO
3
are shaded in red below:
1. Os should never be mixed with HNO
3
due to
the formation of the very volatile OsO
4
.
2. Cl is oxidized to molecular Cl
2
which is volatile
and adsorbs on plastic.
3. Br and I are oxidized to molecular Br
2
and I
2
which adsorb onto plastic.
4. Dilutions of Hg and Au in HNO
3
below 100
ppm should be stored in borosilicate glass due to
Hg
+2
adsorption on plastic.
5. Not soluble above concentrations of 1000 μg
mL.
6. Trace levels of HCl or Cl- will form AgCl,
which will photoreduce to Ag
0
.
F
denotes that the element can be diluted in HNO
3
if complexed with F-.
Cl
denotes that the element can be diluted in HNO
3
if complexed with Cl-.
HF
denotes that the element should have excess HF present when diluted with HNO
3
.
T
denotes that the tartaric acid complex can be diluted in HNO
3
.
Hydrochloric Acid Matrices
The use of hydrochloric acid (HCl) is the next most popular acid matrix. HCl is volatile and it is corrosive to the
instrument and it's electronics therefore, exposure should be kept to a minimum.
The elements that can be diluted in HCl are shaded in blue below:
1. Concentrated (35%) HCl will keep up to 100
μg/mL of Ag
+
in solution as the Ag(Cl)
X-(X-1)
complex. For more dilute solutions, the HCl can
be lowered such that 10% HCl will keep up to 10
μg/mL Ag in solution.
NOTE: The Ag(Cl)
X-(X-1)
complex is photosensitive
and will reduce to Ag
0
when exposed to light.
HNO
3
solutions of Ag
+
are not photosensitive.
2. Parts-per-billion (ppb) dilutions of Hg
+2
in HCl
are more stable to adsorption on the container
walls than are dilutions in HNO
3
.
F
denotes that the element is more stable to
hydrolysis if complexed with F-. In the case of Si and Ge the fluoride complex is generally considered a necessity.
















