
S4
ESTRO 36 2017
_______________________________________________________________________________________________
were obtained for immune monitoring. Patients were
evaluated for abscopal responses with baseline and post-
treatment (day 88) PET/CTs. Metastases outside the RT
field were measured on axial CTs in 2 perpendicular
dimensions. The products of the measurements (l x w) in
all abscopal lesions were summed. Baseline and post-
treatment measurements of abscopal lesions were
compared. Abscopal responses were reported as: CR –
complete resolution, PR – decrease in size ≥30%, PD –
increase in size ≥20%, or SD – insufficient shrinkage or
growth to qualify for PR/CR or PD. Toxicities were
reported according to the common terminology criteria for
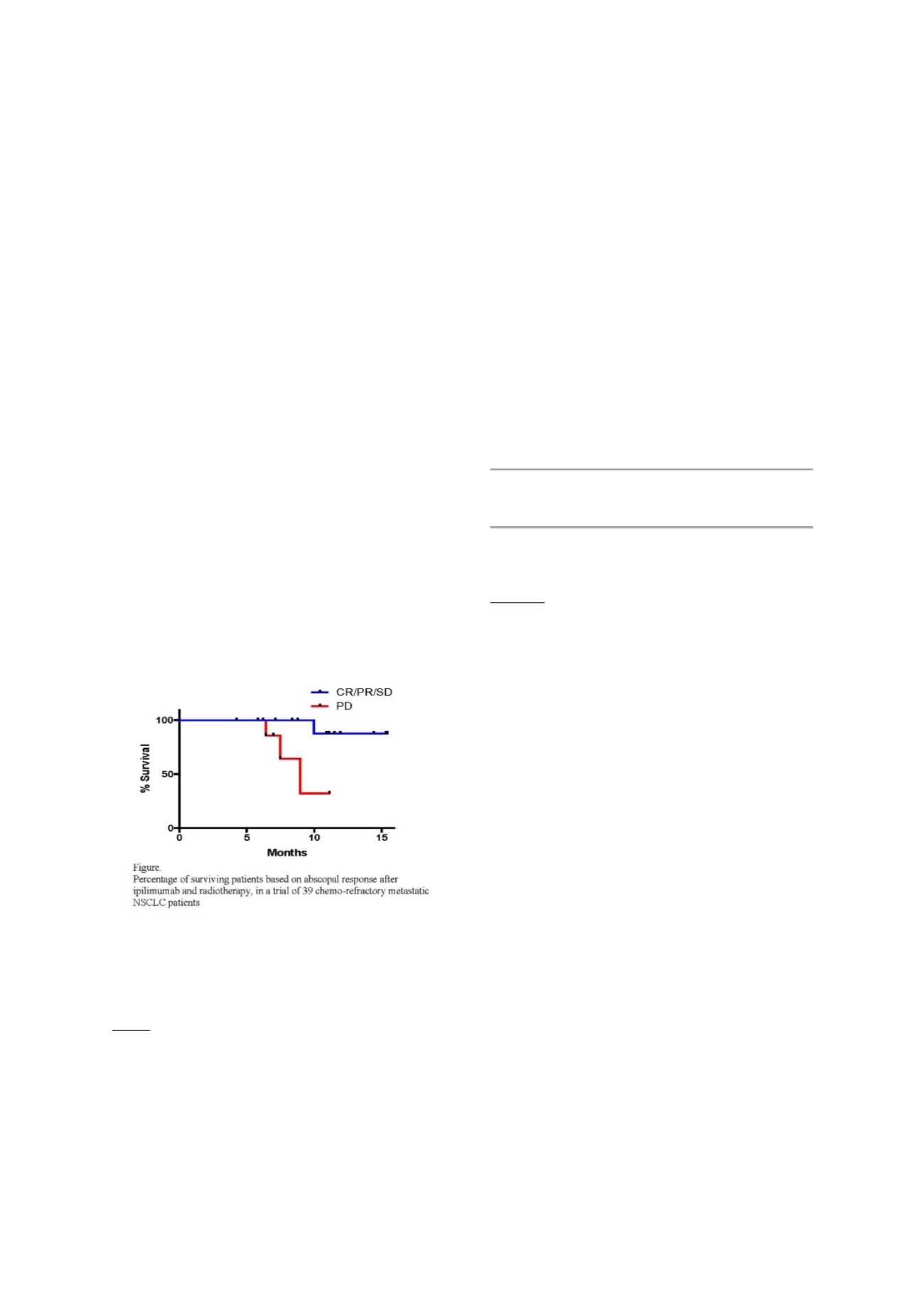
adverse events version 4.0. Thirty-nine patients accrued.
Based on intent to treat abscopal response rate was 18%.
Seven of the 21 patients who completed the 4 cycles of
Ipilimumab had an abscopal response (33%). At median
follow up of 16 months the achievement of an initial
abscopal response to the regimen remains associated with
better survival (HR=9.174, log-rank test p=0.061) (Figure).
Expression of PD-L1 >10% in pre-treatment tumor biopsies
was observed among patients who achieved complete and
partial abscopal responses, suggesting that T cells
activated by RT and CTLA-4 blockade can reject PDL-1
positive tumors. Finally, marked changes in peripheral
blood T cell clonality 3 weeks after combined
treatment was demonstrated in the patients who
developed abscopal responses but not in non responders.
In a patient with complete response and sufficient tumor
material for TCR Vbeta deep sequencing a T cell clone
found in the tumor at low levels before treatment
appeared in the blood at 3 weeks and persisted after
completion of treatment. In conclusion, objective
abscopal responses were common in NSCLC patients
treated with local RT and ipilimumab, independently from
initial PD-L1 expression. Immunologic characterization of
tumor infiltrating lymphocytes and tumor antigen-specific
T- and B-cell responses in treated patients is ongoing
[clinicaltrials.gov NCT02221739].
SP-0013 The use of novel technologies (e.g. protons) in
NSCLC
Z. Liao
1
1
UT MD Anderson Cancer Center Radiation Physics,
Houston- TX, USA
Technological innovations during the last 2 decades has
revolutionized photon radiotherapy and resulted in
improved clinical outcomes of lung cancer patients in
terms of reduced radiation toxicity and increased tumor
control and survival. These innovations include intensity
modulated radiation, volumetric modulated arc therapy,
image guided radiation therapy. Proton therapy can offer
substantial clinical advantage over the photon therapy
because protons have unique depth-dose characteristics
that can potentially significantly reduce normal tissue
doses proximal and distal to the target volume and allow
escalation of tumor doses. Lung cancer is one of the many
cancer types that could benefit from proton therapy.
Treatment planning and plan evaluation of PSPT and IMPT
demonstrated significant reduction of mean lung dose and
mean heart dose. Results from retrospective studies from
our institution and others were promising. However, the
results of the first adaptive randomization trial comparing
passively scattered proton therapy (PSPT) with intensity-
modulated (photon) radiotherapy (IMRT), both with
concurrent chemotherapy, for patients with inoperable
lung cancer showed no statistically significant differences
in the primary endpoint (radiation pneumonitis or local
failure) were found between IMRT vs. PSPT. Higher-than-
expected RP in the PSPT arm may have reflected larger
high-dose volumes and a steeper learning curve for
practitioners treating with protons. The results also
suggest that the dose constraints used to guide IMRT may
not be applicable for protons. The most current evidence
and challenges in using protons for lung cancer will be
reviewed in this symposium.
Symposium with Proffered Papers: Radiotherapy plus
immunotherapy combination: rationale and results so
far
SP-0014 In situ Cancer Vaccines: Tumor destruction
and immune stimulation for local and systemic tumor
control.
G. Adema
1,2
, M. Den Brok
1,2
, R. Van den Bijgaart
1,2
, M.
Wassink
1,2
, M. Hoogenboom
2
, J. Bussink
1,2
, J. Futterer
2
1
Radiotherapy & OncoImmunology lab, Dept. of
Radiation Oncology, Nijmegen, The Netherlands
2
RadboudUMC, Nijmegen, The Netherlands
In situ Cancer Vaccines: Tumor destruction and immune
stimulation for local and systemic tumor control.
T
umor ablation techniques are successfully applied for the
treatment of cancer. These techniques use e.g. radiation,
heat or cold to locally destruct often inoperable tumor
masses. After ablation tumor antigens become instantly
available for antigen presenting cells, and the procedure
itself creates an inflammatory environment that will
effect the immune system and hence anti-tumor
immunity. Despite the reported enhanced presence of key
immunological correlates, strong immune responses
(abscopal effect) have only rarely been observed after
tumor ablation as monotherapy. As a result, patients often
succumb to tumor micro-metastases being already present
prior to treatment. Therefore, there is a strong need for
systemic adjuvant therapy, like immunotherapy, targeting
these residual tumor cells. To obtain robust immunity we
showed in murine models that ablation should be
combined with immune modulation using adjuvants or
immune checkpoint blockade mAbs. Moreover, we showed
the involvement of mature lymph node dendritic cells,
actively scavenging and cross-presenting antigens from
the tumor. This yielded long-lasting memory immune
responses and protection against a tumor rechallenge and
even pre-existing metastasis. It is currently not fully
understood how immune responses following radiotherapy
can be optimally initiated and regulated. More
importantly, the most effective immune stimulating
compounds for Immuno-radiotherapy are not known. We
will discuss our ongoing research program to uncover the
potency of RT in combination with immune based
strategies to eliminate tumor cells and their
microenvironment for both local and systemic tumor
control.


















