
standard solution and 2 g ISTD standard solution into a 50
mL flask. Dilute to volume with water and accurately weigh
the solution. Cap and mix well. Transfer to an autosampler
vial and cap.
(
5
) Level 5 calibration standard. - Accurately weigh 26 g
standard solution and 2 g ISTD standard solution into a 50
mL flask. Dilute to volume with water and accurately weigh
the solution. Cap and mix well. Transfer to an autosampler
vial and cap.
(
6
) Level 6 calibration standard. - Accurately weigh 40 g
standard solution and 2 g ISTD standard solution into a 50
mL flask. Dilute to volume with water and accurately weight
the solution. Cap and mix well. Transfer to an autosampler
vial and cap.
F. Sample preparation and Extraction
(
a
)
Sample weighing. –
(
b
) Accurately weighed the extraction vessels.
(
1
) For dry blended/nonhomogeneous powder samples,
transfer 25 g, accurately weighed to a 250 mL volumetric
flask. Dissolve using warm distilled water ~40°C, cool, and
make up to 250 g accurately weighed with distilled water.
Transfer 10 - 15 g accurately weighed of reconstituted sample
to the extraction vessels.
(
2
) For wet blended homogeneous powder samples, transfer 2
- 3 g, accurately weighed directly to the extraction vessels.
(
3
) For ready-to-feed samples or concentrated liquid
products, transfer 5 to 10 g, accurately weighed of thoroughly
agitated sample directly to the extraction vessel.
(
4
) For dry food or animal feed samples, transfer 5 - 10 g,
accurately weighed of sample directly to the extraction
vessel.
(
5
) For wet food or animal feed samples, transfer 10 - 15 g,
accurately weighed of sample directly to the extraction
vessel.
(
6
) For nutritional supplements, transfer 1 – 1.5 g, accurately
weighed of sample directly to the extraction vessel.
(
c
) Ekstraction. –
(
1
) For liquid samples no added water is necessary. For wet
samples add 5 mL of water. For dry samples add 10 ml of
water.
(
2
) Add 100 ml of Methanol (HPLC-grade), and cap.
(
3
) Mix by shaking until well dispersed. Place flask on
shaking table at 37°C for 12 h. adjust shaking to obtain good
mixing.
(
4
) Cool to ambient temperature.
(
5
) Accurately weigh the extraction vessel.
(
6
) Transfer part of the extraction liquid to a 50 mL
centrifuge tube, and cap.
(
7
) Centrifuge the extraction liquid to obtain a clear liquid,
(approximately 200 rpm for 10 min).
(
d
) Determination of free carnitine -
(
1
) For high concentration samples a dilution step with water
should be performed before addition of the internal standard.
(
2
) Accurately weigh the digestion vessels.
(
3
) Transfer 1 – 5 g accurately weighed, of the clear
extraction liquid to the digestion vessels.
(
4
) Add 10 g accurately weighed ISTD standard solution to
the digestion vessels.
(
5
) Dilute to 250 mL volume with water and accurately
weight the solution. Cap and mix well. Transfer to an auto-
sampler vial and cap.
(
e
) Determination of Total carnitine and –
(
1
) For high concentration samples a dilution step with water
should be performed before addition of the internal standard..
(
2
) Accurately weigh the digestion vessels.
(
3
) Transfer 1 – 5 g accurately weighed, of the clear
extraction liquid to the digestion vessels.
(
4
) Add 10 g accurately weighed ISTD standard solution to
the digestion vessels.
(
5
) Add 3 mL of 2M Potassium hydroxide solution, and 47
mL of water, close with stopper.
(
6
) Hydrolyse in oven at 102°C for 4 h.
(
7
) Cool to ambient temperature.
(
8
) Add 4 mL 1.6M Hydrochloric acid
(
9
) Dilute to 250 mL volume with water and accurately
weight the solution. Cap and mix well. Transfer to an
autosampler vial and cap.
G. Instrument operation conditions
(
a
) LC. – Isocratic run 0-2 min. 98% A; 2% B. Flow 0,5
mL/min; injection volume, 2 μL; column temperature, 25°C;
autosampler temperature, ambient.
(
b
) MS/MS.—Ionization mode, positive-ion electrospray
ionization (ESI+);Curtain gas, 30 psi;Collision gas, Med;
Ionspray voltage , 5.5 kV; source temperature, 700°C; Ion
Source Gas 1, 65 psi;Ion source gas 2, 65 psi.
(
c
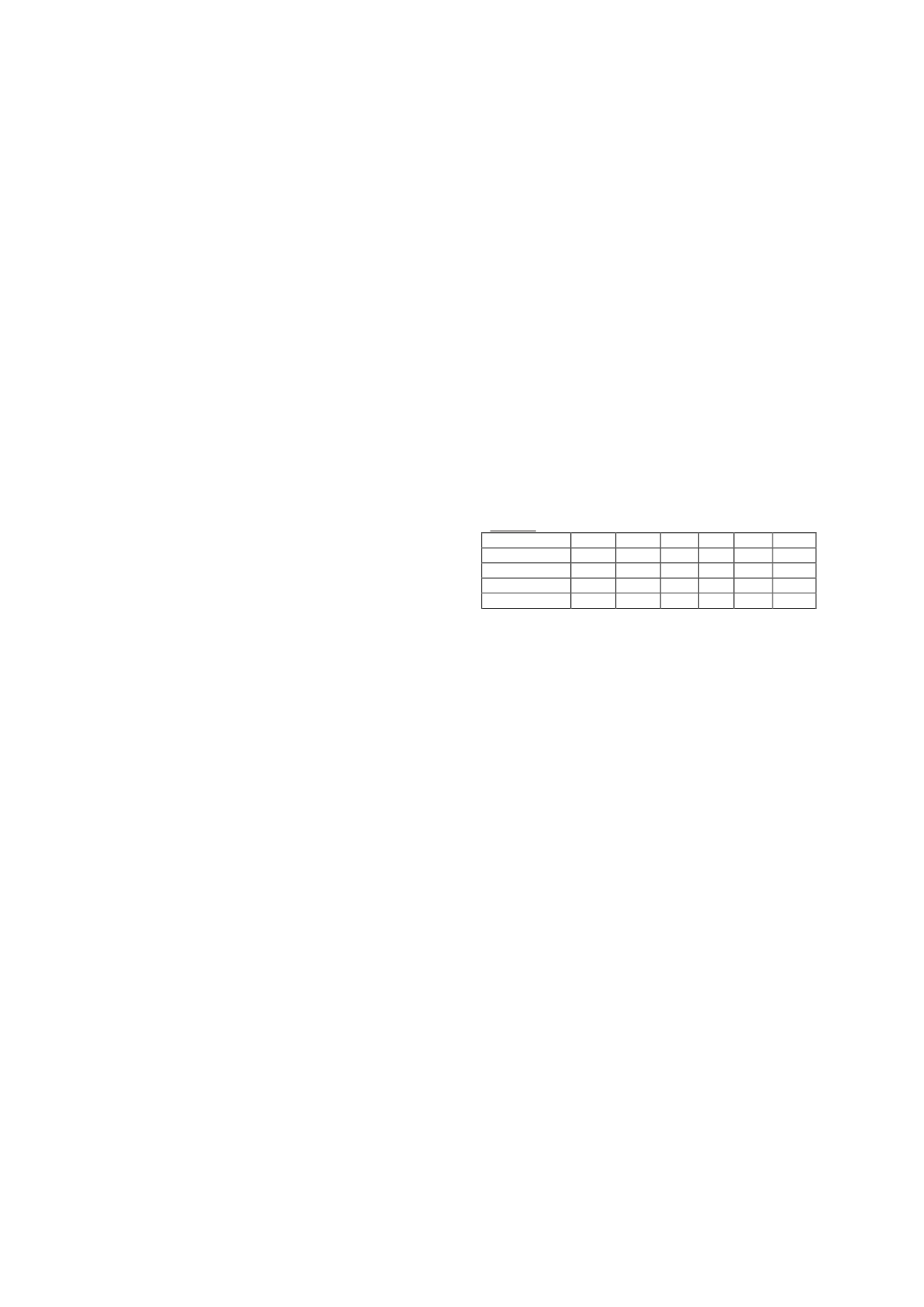
) Parameters for MS/MS measurement see table 1.
Table 1.
Analyte
Q1
a
Q3
b
DP
c
EP
d
CE
e
CXP
f
L-carnitine
162.2 103.1 36.0
5.0 19.0
4.0
L-carnitine
162.2
85.1 36.0
5.0 25.0
4.0
L-Carnitine-d
3
165.2 103.1 36.0
5.0 19.0
4.0
L-Carnitine-d
3
165.2
85.1 36.0
5.0 25.0
4.0
a
Q1 = Quadrupole mass filter 1.
b
Q3 = Quadrupole mass filter 3.
c
DP = Declustering potential.
d
EP = Entrance potential.
e
CE = Collision energy.
f
CXP = Collision cell exit potential.
(
d
) Data acquisition was done in the multiple-reaction
monitoring (MRM) mode.
(
e
) Quantitation of L-carnitine.—The MRM chromatogram
for L-carnitine is a sum of signals for the transitions m/z 162
> 103 and 162 > 85. Likewise, the MRM chromatogram for
the ISTD is a sum of signals for the transitions m/z 165 > 103
and 165 > 85. Analyst automatically calculates a response
ratio, which is defined as the L-carnitine peak area divided by
the ISTD peak area.
(
f
) UPLC analysis.—After verifying equilibration of the
UPLC system, inject the working standards (L1 to L6)
followed by a reagent blank, control sample, and sample
extracts.
(
g
) Inject working standards after approximately every 20
sample extraction, injected after the analysis of the last
sample extract. Notes: Calibration curves must have a
correlation coefficient r
2
of >0.990.
H. Calculation of results
(
a
) Manual calculations.—
(
1
) Plot each calibration standard response ratio versus its
corresponding concentration to obtain calibration curves for
L-carnitine. Apply a nonweighted linear regression to the
data, and obtain an equation for the best-fit line.
Carn-06
FOR ERP USE ONLY
DO NOT DISTRIBUTE










