
S217
ESTRO 36 2017
_______________________________________________________________________________________________
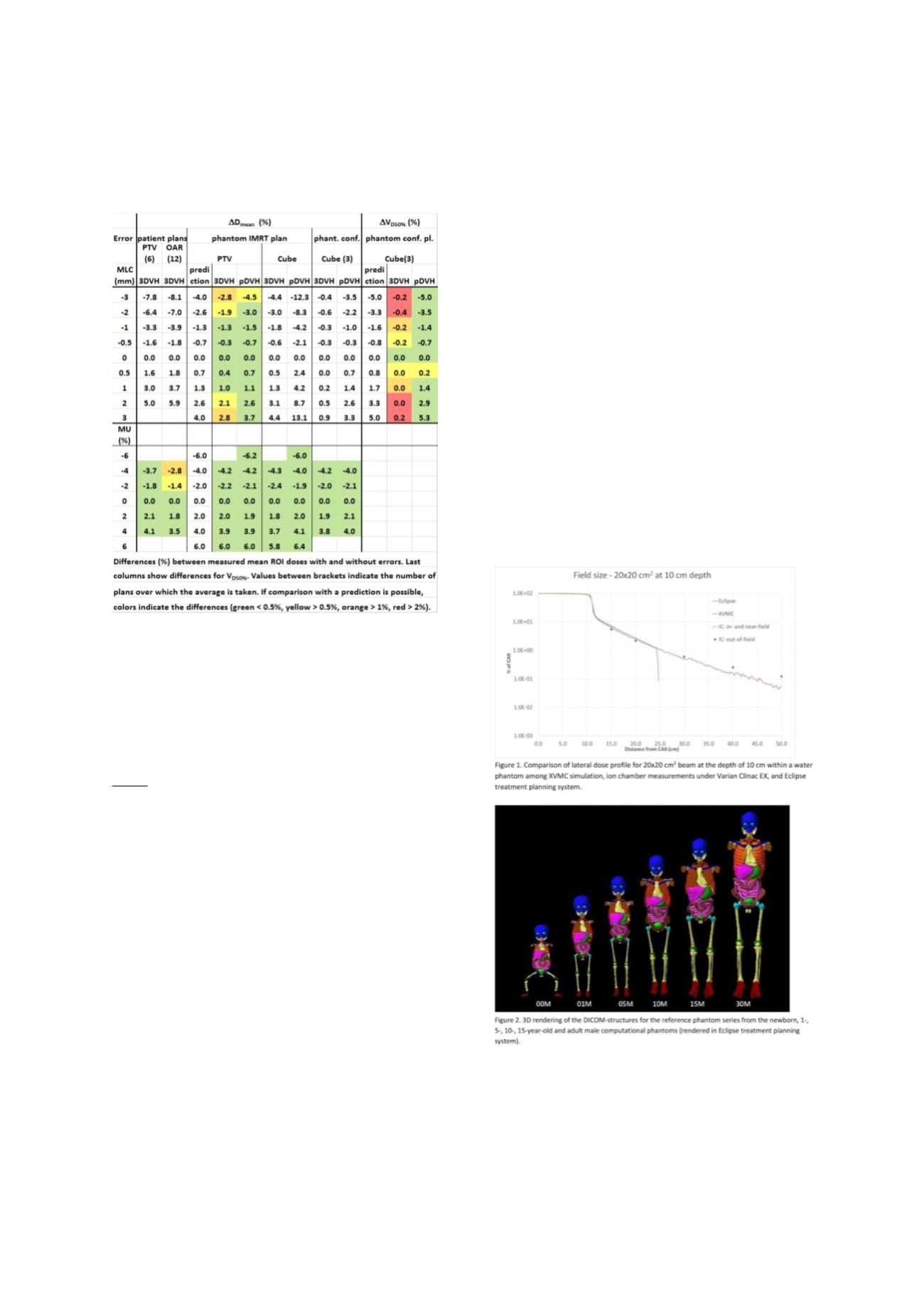
for MLC errors, especially for the conformal
plans and OARs (Table).
4.
Comparing with the predictions, it appears that
3DVH
tends to underestimate the effect of MLC
errors, whereas
pDVH
performs more
accurately (Table).
Conclusion
We developed a procedure to verify DVH measurements
for individual patient QA.
3DVH
has similar gamma results
as 2D ArcCheck for clinical cases. Phantom studies
however indicate that
3DVH
can underestimate the
dosimetric effect of MLC errors, where EPID based
pDVH
performs better.
PV-0416 Novel methods for normal tissue dose in
epidemiological studies of second cancer in
radiotherapy
C. Lee
1
, J.W. Jung
2
, C. Lee
3
, M.M. Mille
1
, E. Mosher
1
, C.
Pelletier
2
, G. Kuzmin
1
1
National Cancer Institute, Division of Cancer
Epidemiology and Genetics, Bethesda, USA
2
East Carolina University, Department of Physics,
Greenville, USA
3
University of Michigan, Department of Radiation
Oncology, Ann Arbor, USA
Purpose or Objective
Dose estimation of normal tissues located outside
treatment beam fields is one of the crucial components in
retrospective epidemiological studies of late effects in
radiotherapy patients but there are three challenges.
First, dosimetry methods for out-of-field normal tissues
are not well established compared to in-field. Second,
radiological images of patient anatomy are not commonly
available. Third, even if patient images are available,
contouring several normal organs of interest would take
substantial effort for large-scale patient cohorts. We have
developed computational solutions: to calculate normal
tissue doses for out-of-field region, which was validated
by experiment; to construct a realistic surrogate anatomy
by using computational human phantoms; and to
automatically contour major organs of interest on patient
images.
Material and Methods
We employed XVMC computer code for Monte Carlo
radiation transport with enhanced computation speed. We
adjusted the virtual source models built in XVMC to match
out-of-field dose profile measured in a water phantom.
For the cases where patient images are not available, we
converted a library of existing computational human
phantoms with the range of age and body size in voxel
format into Digital Imaging and Communications in
Medicine (DICOM)-images by translating material
composition and density to Hounsfield Unit. We then
converted the organ structures in the voxel phantoms into
DICOM-structures and tested the resulting DICOM
phantoms with multiple treatment planning systems.
Finally, for the cases where patient images may be
available for a large number of patients, we developed
methods to automatically contour the heart and its
substructures, as a start, by using an atlas library derived
from 60 adult male and female patients.
Results
First, the lateral dose profiles computed from XVMC and
the water phantom matched well within 15% (<10 cm from
field edge) and 40% (> 10 cm and <30 cm from the field
edge)(Figure 1). Second, a set of DICOM-images and
structures was generated from the pediatric and adult
reference and non-reference phantom series (Figure 2).
We validated the DICOM phantoms by comparing density
and volume of selected organs between the original
phantom and Eclipse showing a good agreement less than
5% and 0.1% for density and volume, respectively. Finally,
we confirmed that the auto-contoured heart and
manually-contoured heart for 30 adult male patients show
the Dice coefficients up to 91% for the total heart and up
to 84% for the left ventricle.
Conclusion
The computational methods established in this study will
be useful for the reconstruction of normal tissue dose to
support epidemiological studies of second cancer in
cancer survivors treated by radiotherapy, where
radiological images of patients may or may not be
available.
PV-0417 Validation of an analytical peripheral photon
dose model for FFF modality


















