
S223
ESTRO 36 2017
_______________________________________________________________________________________________
Results
SMART was delivered in 45 fractions in nine pts (4F, 5M;
ages 55-87 yrs) with LAPC. Two pts had biliary stents. All
pts were able to complete the BH delivery. Median
duration of the SMART delivery was 54 min (range 42-73).
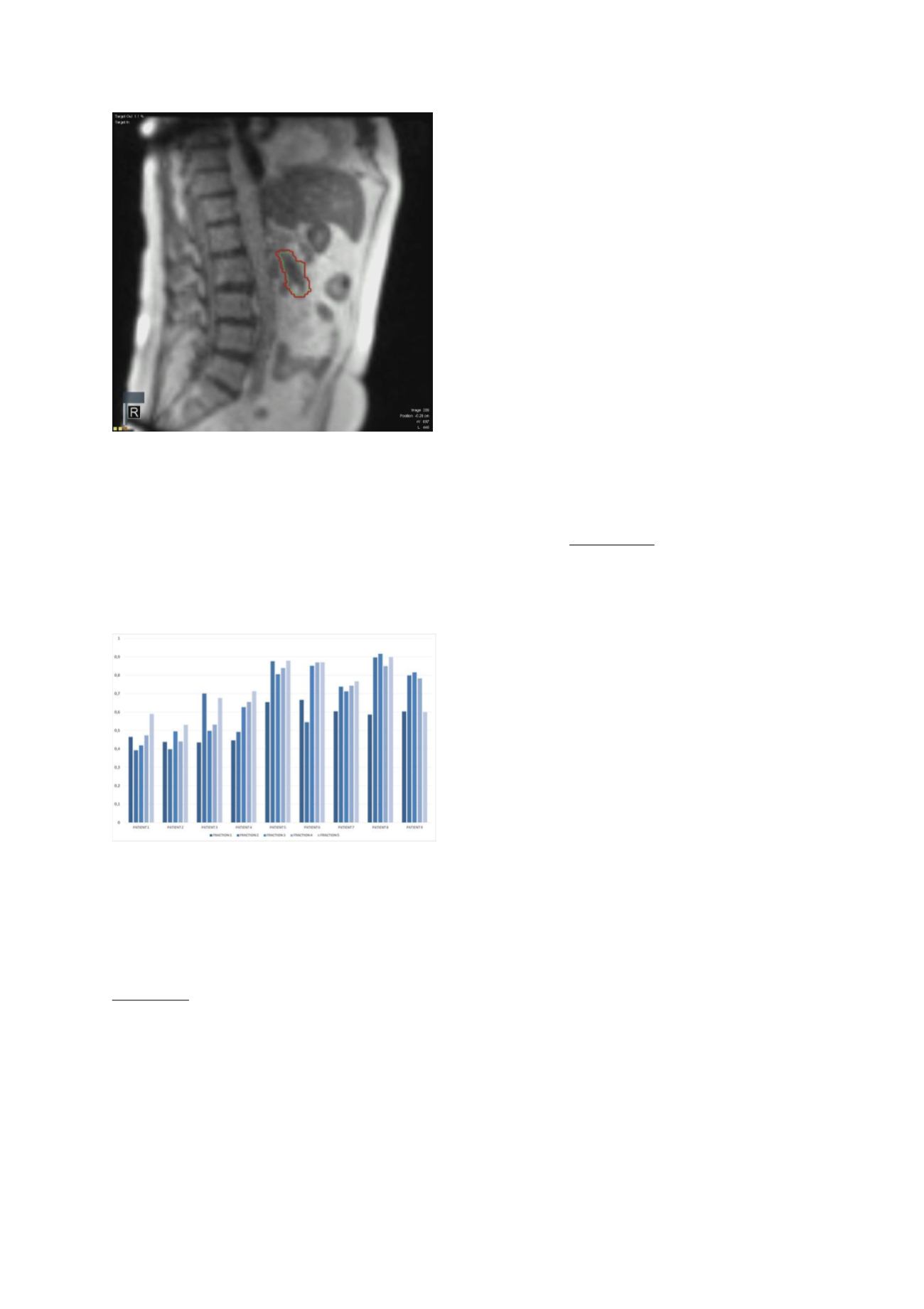
With the video-feedback method, median gated treatment
efficiency (ratio between actual beam-on time and
delivery time) was 0.66 for all fractions, ranging from
0.40-0.92 (Fig 2). Pt follow-up is still limited, but early
results show no grade ≥3 acute toxicity. Prospectively-
scored patient reported outcomes revealed maximum
Grade 2 fatigue and nausea in, respectively, 6 pts and 1
pt.
Conclusion
SMART is novel treatment approach for LAPC that requires
no placement of fiducials, and is well tolerated, even by
elderly pts and those with stents. Initial experience
revealed that delivery within a one hour time-frame per
fraction is feasible. Updated clinical follow-up data will
be presented.
OC-0426 Adjuvant chemoradiation in pancreatic
cancer: impact of radiotherapy dose on survival
A.G. Morganti
1
, M. Falconi
2
, G.C. Mattiucci
3
, A. Arcelli
1,4
,
F. Bertini
1
, A. Farioli
5
, A. Guido
1
, M.C. Di Marco
6
, L.
Fuccio
5
, S. Alfieri
7
, F.A. Calvo
8
, B.W. Maidment 3rd
9
, R.C.
Miller
10
, M. Reni
11
, G. Macchia
12
, F. Deodato
12
, S. Cilla
13
,
G. Di Gioia
12
, F. Cellini
3
, V. Valentini
3
1
University of Bologna- S. Orsola-Malpighi Hospital,
Radiation Oncology Center- Department of
Experimental- Diagnostic and Speciality Medicine- DIMES,
Bologna, Italy
2
San Raffaele Hospital, Department of Surgery-
Pancreatic Surgery Unit, Milano, Italy
3
Università Cattolica S. Cuore, Department of
Radiotherapy, Rome, Italy
4
Ospedale Bellaria, Radiotherapy Department, Bologna,
Italy
5
University of Bologna, Department of Medical and
Surgical Sciences - DIMEC, Bologna, Italy
6
University of Bologna- S. Orsola-Malpighi Hospital,
Department of Oncology, Bologna, Italy
7
Università Cattolica S. Cuore, Department of Surgery,
Rome, Italy
8
Hospital General Universitario Gregorio Marañón-
Complutense University, Department of Oncology,
Madrid, Spain
9
University of Virginia- Charlottesville, Department of
Radiation Oncology, VA, USA
10
Mayo Clinic, Department of Radiation Oncology,
Rochester, USA
11
S. Raffaele Scientific Institute, Department of
Oncology, Milano, Italy
12
Fondazione Giovanni Paolo II, Unit of Radiotherapy-
Unit of General Oncology, Campobasso, Italy
13
Fondazione Giovanni Paolo II, Unit of Medical Physics,
Campobasso, Italy
THIS ABSTRACT FORMS PART OF THE MEDIA PROGRAMME
AND WILL BE AVAILABLE ON THE DAY OF ITS
PRESENTATION TO THE CONFERENCE.
OC-0427 Prediction models in rectal cancer: an
update of a pooled analysis of 3770 randomized
patients
V. Valentini
1
, C. Masciocchi
1
, J. Van Soest
2
, G. Chiloiro
1
,
E. Meldolesi
1
, M. Gambacorta
1
, J. Gerard
3
, S. Ngan
4
, J.
Bosset
5
, A. Sainato
6
, A. Damiani
1
, N. Dinapoli
1
, P.
Lambin
2
, A. Dekker
2
, C. Roedel
7
1
Università Cattolica del Sacro Cuore -Policlinico A.
Gemelli, Radiation Oncology Department, Rome, Italy
2
Maastricht University Medical Center, Radiation
Oncology MAASTRO-GROW School for Oncology and
Development Biology, Maastricht, The Netherlands
3
Unicancer- Centre Antoine Lacassagne, Radiotherapy,
Nice, France
4
Peter MacCallum Cancer Centre, Division of Radiation
Oncology, Melbourne, Australia
5
Besançon University Hospital J Minjoz, Radiation and
Oncology, Besançon, France
6
Azienda ospedaliera Universitaria Pisana, Radiotherapy,
Pisa, Italy
7
Goethe University Frankfurt, Radiotherapy and
Oncology, Frankfurt am Main, Germany
Purpose or Objective
In the last years, several prognostic and predictive models
(PMs) for locally advanced rectal cancer (LARC) patients
(pts) have been developed. Aim of this study was to
update the previous PMs [1] developed for local
recurrence (LR), distant metastases (DM) and overall
survival (OS) at 2, 3, 5 and 10 years based on a more
copious pooled set of LARC pts.
Material and Methods
The PMs were developed using the data of the following
LARC trials: Accord 12/0405, EORTC 22921, FFCD 9203,
CAO/ARO/AIO-94, CAO-ARO-AIO-04, INTERACT, I-CNR-RT
and TROG 01.04. Pts were selected applying the following
exclusion criteria: neoadjuvant and adjuvant oxaliplatin
based chemotherapy, no surgery procedure, short-course
radiotherapy and no neoadjuvant radiotherapy. As the
current pooled dataset contains different trials, we used
20% of the data (stratified per trial) as a validation
dataset. Due to variable influence over time, a logistic
regression model was used. Follow-up times (2, 3, 5 and
10 years) for the survival outcomes (LR, DM and OS) were
used as the model outcome. Variable selection was
performed using a stepwise Akaike's information criterion
(AIC) feature selection to determine the optimal subset of
covariates and nomograms developed as a visual
representation. The nomogram shows only significative
covariates (p<=0.01). According to the TRIPOD [2], all Pms


















